Choose your sales office
PRODUCT
Serology
Complementary immunological test to the direct detection of urogenital mycoplasma.
The SEROLOGY kit enables the quantitative detection in serum of the antibodies against the principal urogenital mycoplasma species, Ureaplasma urealyticum (U.u.) and Mycoplasma hominis (M.h.). Given their lack of immunogenicity, serological testing of mycoplasmas does not replace direct diagnosis from patient specimens but is a pertinent complementary test. The serologic test for urogenital mycoplasmas is especially use to diagnose a deep genital infection, like salpingitis or prostatitis. The technique of metabolic inhibition using lyophilized reagents and reference strains is the most appropriate technique for use by non-specialized laboratories. More than being the fastest technology available for Mycoplasma serology testing, our SEROLOGY test offers:
- Simple methodology with no prior treatment of serum
- High sensitivity and specificity
- Fast results for clinicians, available within 24 hours
- Visual colorimetric method, without expert interpretation
- Control serum standard included in the kit

Image Gallery
Benefits
Intended use
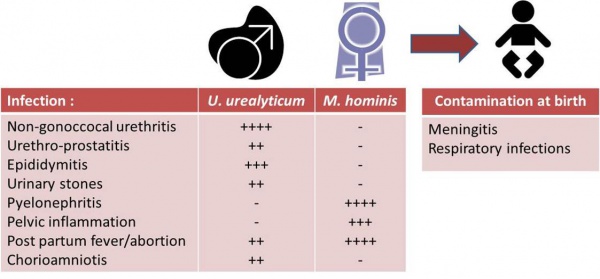 Conventional diagnosis is based upon culture on A7 agar plates followed by microscopical identification of U.u. (sea urchin shaped) or M.h. (fried-egg shaped) colonies. Since both U.u. and M.h. are commensal, infection can only be diagnosed through the determination of the pathological threshold, followed by precise enumeration. Given their lack of immunogenicity, serological testing of mycoplasmas does not replace direct diagnosis from patient specimens. The serologic test for urogenital mycoplasmas is especially used to diagnose a deep genital infection, like salpingitis or prostatitis. The technique of metabolic inhibition using lyophilized reagents and reference strains is the most appropriate technique for use by non-specialized laboratories.
Conventional diagnosis is based upon culture on A7 agar plates followed by microscopical identification of U.u. (sea urchin shaped) or M.h. (fried-egg shaped) colonies. Since both U.u. and M.h. are commensal, infection can only be diagnosed through the determination of the pathological threshold, followed by precise enumeration. Given their lack of immunogenicity, serological testing of mycoplasmas does not replace direct diagnosis from patient specimens. The serologic test for urogenital mycoplasmas is especially used to diagnose a deep genital infection, like salpingitis or prostatitis. The technique of metabolic inhibition using lyophilized reagents and reference strains is the most appropriate technique for use by non-specialized laboratories.Simple protocol
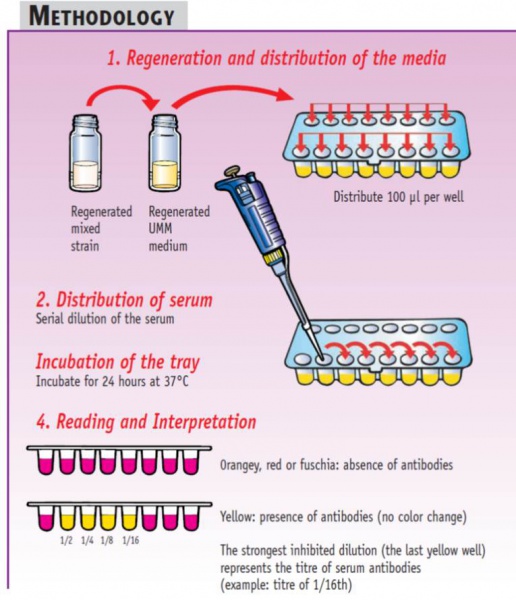
Easy-to-read and eay-to-interpret results
“
Reagents and Material
- UMMt:
- Vial of 2 mL of liquid mycoplasma base medium
- Quantty: 14
- UMMlyo:
- Vial of lyophilized mycoplasma growth medium (+ 2 mL UMMt)
- Quantity: 14
- U.u/M.h:
- Vial containing lyophilized U. urealyticum and M. hominis strains
- Quantity: 14
- SEROLOGY tray:
- 20-well tray wrapped individually in an aluminum sachet
- Quantity: 14
- Positive Control:
- Vial containing a lyophilized solution of known titre for verification of the reagents
- Quantity: 1
Stability and Storage
The reagents, stored at 2 to 8 °C in their original packaging, are stable until the expiry date shown on the kit.
Performance
A comparative study was carried out with the SEROLOGY reagent and the MYCOKIT SERO reagent of PBS ORGENICS on 100 routinely tested human serum samples (64 serum samples negative for anti U.u. and anti-M.h. antibodies, 28 serum samples positive for anti- U.u. and anti-M.h. antibodies, 6 serum samples positive for anti-U.u. antibodies and negative for anti-M.h. antibodies, and 2 serum samples negative for anti-U.u. antibodies and positive for anti-M.h. antibodies). Both methods are based upon the same principle of metabolic inhibition. Reading of the SEROLOGY tray was carried out after incubation for 48 hours:
- For the detection of anti-U.u. antibodies, the sensitivity is 88.2% and the specificity is 100%. The 4 false negatives corresponded to a serum sample positive at dilutions of 1/2 and 1/4.
- For the detection of anti-M.h. antibodies, the sensitivity is 96.6% and the specificity is 98.5%.
- For the determination of the antibody titre, the results of the study indicated a concordance of 92.5%, irrespective of the magnitude of the difference in the titre, and a concordance of 97.5% for titres to within one dilution.
Material required but not provided
- Sterile pipettes
- Sterile distilled water
- Paraffin oil
- Incubator calibrated to 37 °C
- Waste container for contaminated waste
Manufacturer: Elitech Microbio.
Product(s) intended for healthcare professionals.
Read the instructions on the label and/or instructions for use of the product(s).
![]()
Let us help you
For general inquiries, please use the links to the right. Click Contact to complete a brief online form, or click Support for general phone and email information. Someone will be in touch with you soon.