Choose your sales office
PRODUCT
A7 Agar
Agar plates for specific culture, enumeration and morphological identification of urogenital mycoplasma.
The A7 Agar enables the culture, semi-quantitative enumeration and the morphological identification (solid method) of Ureaplasma urealyticum (U.u.) and Mycoplasma hominis (M.h.) from endocervical, urethral, urinary and sperm samples, as well as gastric secretions and other samples likely to contain urogenital mycoplasma infection.
| Reference | Name | Quantity |
| 00090 | A7 Agar | 8 tests |
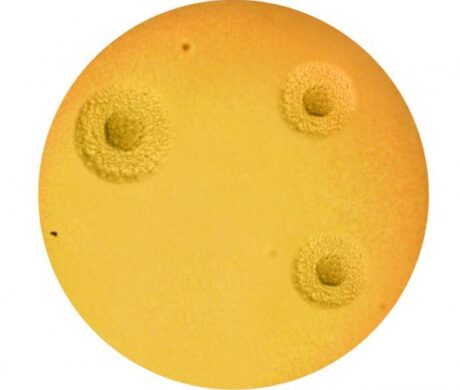
Image Gallery
Benefits
Mycoplasmas are the smallest and simplest of the procaryotypes capable of self-reproducing (0.15 to 0.25 μm). They differ from other bacteria in their lack of a cell wall and hence a natural resistance to ß-lactams. Since mycoplasmas are relatively fragile, they will only grow in acellular cultures in the presence of various growth factors and at a constant temperature of 35 to 37 °C. Most human mycoplasmas are commensal. Of the 9 species that have been isolated from the urogenital tract, Ureaplasma urealyticum and Mycoplasma hominis are the most commonly found. U.u. and M.h. are sexually transmitted and can be pathogenic. Respiratory infections or meningitis can occur in the neonate as a result of contamination from the genital tract at birth. In adults, the infections caused by U.u. and M.h. are described in the table below: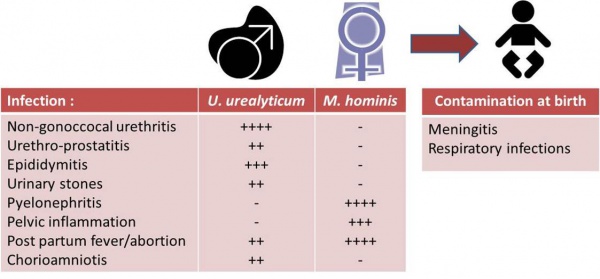
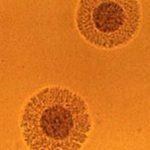
Conventional diagnosis is based upon culture on A7 agar plates followed by microscopical identification of U.u. (sea urchin shaped) or M.h. (fried-egg shaped) colonies. Since both U.u. and M.h. are commensal, infection can only be diagnosed through the determination of the pathological threshold, followed by precise enumeration. Also discover our MYCOFAST® trays for a complete diagnosis including precise enumeration and susceptibility testing to antibiotics.
Principle
The mycoplasmas are relatively fragile organisms that will only multiply in the presence of numerous growth factors. They are facultative anaerobes, demanding in their requirement for sterols. They metabolize sugars or arginine (Mycoplasma hominis) or urea (Ureaplasma urealyticum) in order to provide for their energy requirements. The A7 Agar is a modified Shepard medium, containing serum, peptones, yeast extract and a mixture of vitamins. It is lacking in sugar, but contains urea and arginine as a source of energy. The agar is selective due to the addition of antibiotic and antifungal drugs, thereby inhibiting the development of Gram-positive and Gram-negative bacteria, and fungi. The inclusion of magnesium sulphate results in the Ureaplasma urealyticum colonies having a black coloration in the presence of urea. On agar media, the mycoplasma colonies are small and should be identified with the aid of a microscope. It is recommended that as a complement to the identification of mycoplasmas, a solid media method should be combined with the use of a liquid media method. This can be achieved by using the MYCOSCREEN® or MYCOFAST® liquid methods in combination with the A7 Agar method.
Protocol
The colonies should be identified with the aid of an optical microscope (x10 objective), ensuring that the agar plate is read upside down.
Morphological identification
| Ureaplasma urealyticum | Mycoplasma hominis |
 |
|
| Appearance of a brown-black precipitate (variable size, “sea urchin shaped”). The colonies are small. | “Fried egg shaped” appearance. The colonies are larger than those of U. urealyticum. |
Enumeration
Enumeration is performed upon an upside down agar plate with the aid of an optical microscope (x10 objective). The number of colonies per field of view is recorded:
- Less than 1 colony / field:
- 1 to 5 colonies / field: approx. 104 CFU /mL
- 5 to 10 colonies / field: approx. 105 CFU /mL
- 10 to 20 colonies / field: approx. 106 CFU /mL
- > 20 colonies / field: > 106 CFU /mL
CFU: Colony Forming Units
Reagents and material
| Quantity | Description |
| 8 | A7 Agar: 55mm ready-to-use agar plates, individually wrapped in a cellophane packet. |
Stability and storage
- Stored in their original packaging the reagents are stable until the expiry date shown on the packet.
- Do not expose the agar plates to large variations in temperature.
Performance
- The performance of the A7 Agar culture method was evaluated by comparison with the MYCOFAST liquid method. The study was carried out in a hospital on 544 clinical samples (266 sperm samples, 155 endocervical samples, 82 placentas, 19 urethral samples and 22 miscellaneous samples). For 475 samples (88%), of which 140 were positive and 335 were negative, the results were concordant. The discordances concerned 69 samples, of which 8 were positive only with the A7 Agar (1.5%), 48 were positive only with the MYCOFAST® method (8.8%) and 13 were contaminated (2.4%).
- Certain mycoplasma strains originating from contaminated samples could be detected with the A7 Agar.
Material required but not provided
- Sample collection material (Dacron® swabs, cytobrushes, sterile vials for liquid samples)
- MYCOPLASMA Stabilizer (REF 00064)
- UMMt medium (REF 00835) for swab samples
- Pipettes
- Waste container for contaminated waste
- Facilities for reproducing an anaerobic environment
- Incubator calibrated at 35 to 37 °C
- Optical microscope (x10 objective)
Manufacturer: ELITech Microbio.
Product(s) intended for healthcare professionals.
Read the instructions on the label and/or instructions for use of the product(s).
![]()
Let us help you
For general inquiries, please use the links to the right. Click Contact to complete a brief online form, or click Support for general phone and email information. Someone will be in touch with you soon.