Choose your sales office
PRODUCT
ELI.H.A Schistosoma
Indirect hemagglutination test for serodiagnosis of bilharziosis.
ELI.H.A Schistosoma enables the semi-quantitative determination, by indirect hemagglutination, of serum antibodies from patients with bilharziosis caused by Schistosoma mansoni (intestinal form) and Schistosoma hæmatobium (urinary form).
- Cost-effective test
- Easy and ready-to-use
- High sensitivity and specificity
- Fast results for clinicians (2 hours)
- Simple visual reading, without expert interpretation
| Reference | Name | Quantity |
| 66600 | ELI.H.A Schistosoma | 120 tests |
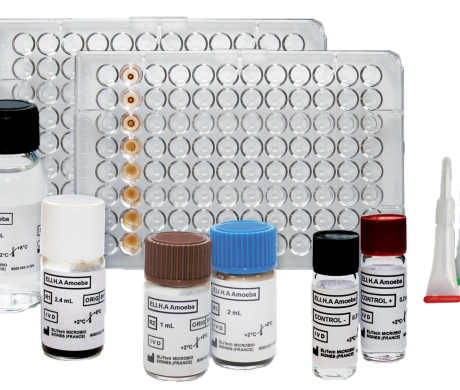
Image Gallery
Benefits
Intended Use
Bilharziosis or schistosomiasis represent a group of parasitic diseases caused by non-segmented flat worms of the genus Schistosoma. In the presence of adult worms, immunity is acquired progressively. However schistosome’s eggs are the pathogenic element of the disease. The disease proceeds in three successive phases: contamination – invasion – disease state. The diagnosis of bilharziosis can be made by eggs identification. The problem is that eggs cannot be detected before the “disease state” phase. Immunological diagnosis, via the detection of antibodies, is thus essential in the initial stages of this disease. ELITech developed the ELI.H.A Schistosoma, an indirect haemagglutination assay, for the semi-quantitative determination of anti-Schistosoma mansoni and anti-Schistosoma hæmatobium antibodies in the serum. It is rapid, easy-to-use and easy-to-read with results available within 2 hours.
Principle
Sheep red blood cells are sensitized with Schistosoma mansoni antigen. Diluted serum is mixed with sensitized sheep red blood cells. If anti-Schistosoma antibodies are present in the serum, sensitized red blood cells will agglutinate, resulting in a cloudy red/brown deposit coating the well. In the absence of specific antibodies, sensitized red blood cells will not agglutinate, resulting in a ring-like deposit at the bottom of the well. Non-sensitized red blood cells are also available in the kit. They ensure the specificity of the reaction by allowing identifying any interference from the natural anti-sheep agglutinins (Forssman heteroantibodies, infectious mononucleosis antibodies…). The reaction is carried out in a U-microplate. Each kit allows 120 tests to be carried out or 20 reactions of 6 dilutions. Results are obtained within 2 hours and are reliable overnight.
Simple Protocol
A single protocol for all our ELI.H.A kits:
- First serum dilution
- Microplate preparation
- Serial dilution of serum and preparation of the ‘serum control’ well
- Addition of sensitized RBC and non sensitized RBC
- Gently mix the plate and let stand 2 hours before reading
The following procedures are therefore valid for all our ELI.H.A range:
Easy-to-read and easy-to-interpret results
| Titer | Interpretation |
| < 1:160 | Non-significant reaction of a progressive infection Can correspond to a previous or treated infection. Renew the test 2 to 3 weeks later and also carry out an electrosyneresis or an immunoelectrophoresis test. |
| ≥ 1:160 | Significant reaction Presumption of an active infection |
Reagents and Material
| Quantity | Description |
| 1 | R1: Vial of 2.4 mL of sensitized red blood cells |
| 1 | R2: Vial of 1 mL of non-sensitized red blood cells |
| 1 | BUF: Vial of 55 mL of phosphate buffer pH 7.2 |
| 1 | R3: Vial of 2 mL of adsorbent |
| 1 | CONTROL +: Vial of 0.2 mL of titrated positive control |
| 1 | CONTROL -: Vial of 0.2 mL of negative control |
| 2 | MICROPLATE: Microplate with a U-bottom |
| 2 | DROPPER: Special dropper |
Material required but not provided
- Automatic pipette(s) with a pipetting volume adapted to the volume that will be measured
- Contaminated waste containers
- Centrifuge
- Hemolysis tubes
Stability and Storage
- The reagents are ready-to-use.
- All the reagents are stored at 2-8°C. Do not freeze.
- The results of the stability study indicate that the product has a stability of 24 months from its manufacture
Performance
| Sensitivity | Specificity |
| 84.4% | 96.9% |
Manufacturer: ELITech Microbio.
Product(s) intended for healthcare professionals.
Read the instructions on the label and/or instructions for use of the product(s).
![]()
Let us help you
For general inquiries, please use the links to the right. Click Contact to complete a brief online form, or click Support for general phone and email information. Someone will be in touch with you soon.

![eliha_methodology[1]](http://www.elitechgroup.com/wp-content/uploads/2015/01/eliha_methodology1.jpg)


![eliha_results[1]](http://www.elitechgroup.com/wp-content/uploads/2015/01/eliha_results1.jpg)