Choose your sales office
PRODUCT
Zika ELITe MGB Kit EUA
Zika ELITe MGB Kit is authorized by the FDA under an EUA for use by authorized laboratories.
- Qualitative real-time PCR test to detect Zika virus in plasma and serum samples
- Indicated for use with the ELITe InGenius® system
- Easy sample-to-result solution
- Validated by major US reference laboratories
- Accurate and sensitive test to support patient management
MGB probes | DSQ probes | Eclipse Dark Quencher | Duplex Stabilizing Quencher | Pleiades probe chemistry | AquaPhluor® Dyes | Superbases
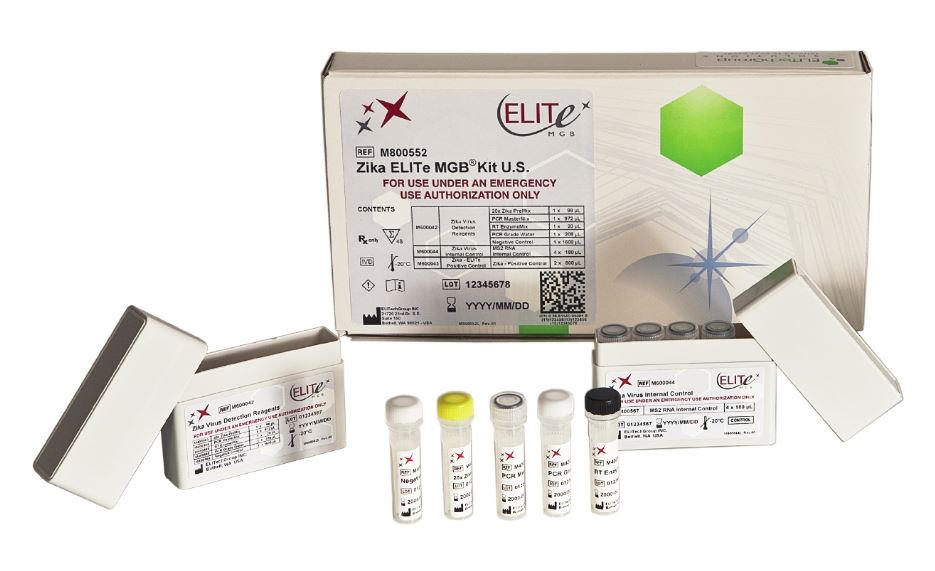
| Catalog number | Product Name | Reactions |
| M800552 | Zika ELITe MGB Kit, EUA | 48 |
Image Gallery
Benefits
Visit egmdx.com to learn more. We offer a full menu of IVD tests, analyte specific reagents (ASRs), and research use only (RUO) materials designed with proprietary chemistries.
Zika ELITe MGB Kit U.S.
• This test has not been FDA cleared or approved;
• This test has been authorized by FDA under an EUA for use by authorized laboratories;
• This test has been authorized only for the detection of RNA from Zika virus and diagnosis of Zika virus infection, not for any other viruses or pathogens; and
• This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of in vitro diagnostic tests for detection of Zika virus and/or diagnosis of Zika virus infection under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Let us help you
For general inquiries, please use the links to the right. Click Contact to complete a brief online form, or click Support for general phone and email information. Someone will be in touch with you soon.

