The W.H.O. estimates that approximately one-quarter of the global population can be defined as anemic, and as such is one of the most common abnormalities identified in routine diagnostic testing/wellness screening (1,2). Iron deficiency, due to inadequate intake or excessive loss, is suspected to be the underlying cause in 50% of all cases (3). Iron deficiency, with or without anemia, has a direct impact on the quality of life. In a report on the Global Burden of Disease Study, iron deficiency anemia was ranked as the fourth highest cause of years lived with disability from a total of 328 diseases and conditions (2). Given this, from a clinical chemistry perspective, the importance of routine iron studies cannot be overstated.
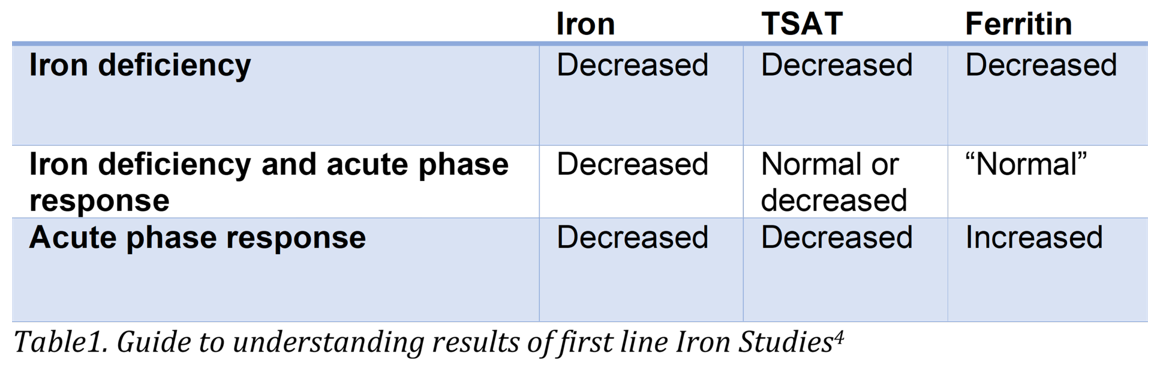
The W.H.O. definition of iron deficiency, based on serum Ferritin concentration, varies according to age, sex, and presence or absence of infection. In the first-line screen of otherwise healthy adults, a serum Ferritin <15 μg/ml is strongly indicative of iron deficiency. As Ferritin is also an acute-phase protein, elevated levels of serum Ferritin may be present in inflammatory conditions, masking iron deficiency, so is typically performed in conjunction with other first-line tests, serum Iron and Total Iron Binding Capacity (TIBC) or Transferrin. With serum iron and TIBC or Transferrin, Transferrin Saturation (TSAT) can be calculated. The results of first-line Iron studies including all three parameters have the benefit of providing a more complete picture than Ferritin only. Performing Fe and TIBC or Transferrin at the same time also avoids inconveniencing the patient with additional blood collections and further costs in some settings (Table 1).
ELITechGroup Clinical Systems has recently added a new Direct TIBC reagent to complete the routine clinical chemistry iron studies panel for the Selectra Family of benchtop systems. The new Direct TIBC has the advantage that it can be fully automated on ELITechGroup Selectra platforms, as no physical separation step is required to remove unbound iron, as is the case with indirect-TIBC methods. The new method is calibrated using Elical 2 (traceable to ERMDA470K and Transferrin), supplied in ready to use liquid stable Selectra System Packaging, has a wide dynamic range and CE marked (IVDR).
For more information on the new Direct-TIBC, please contact clinical.marcom@elitechgroup.com
References:
1. World Health Organization. Iron deficiency anemia: assessment prevention and control. 2001. [cited 2021 Aug 12].
2. S. Numen & K. Kaluza, Systematic review of guidelines for the diagnosis and treatment of iron deficiency anemia using intravenous iron across multiple indications
3. Api O, Breyman C, Cetiner M, et al. Diagnosis and treatment of iron deficiency anemia during pregnancy and the postpartum period: iron deficiency anemia working group consensus report. TJOD. 2015;12(3):173–181.
4. Adapted from the Royal College of Pathologists of Australasia