Choose your sales office
Choose your sales office
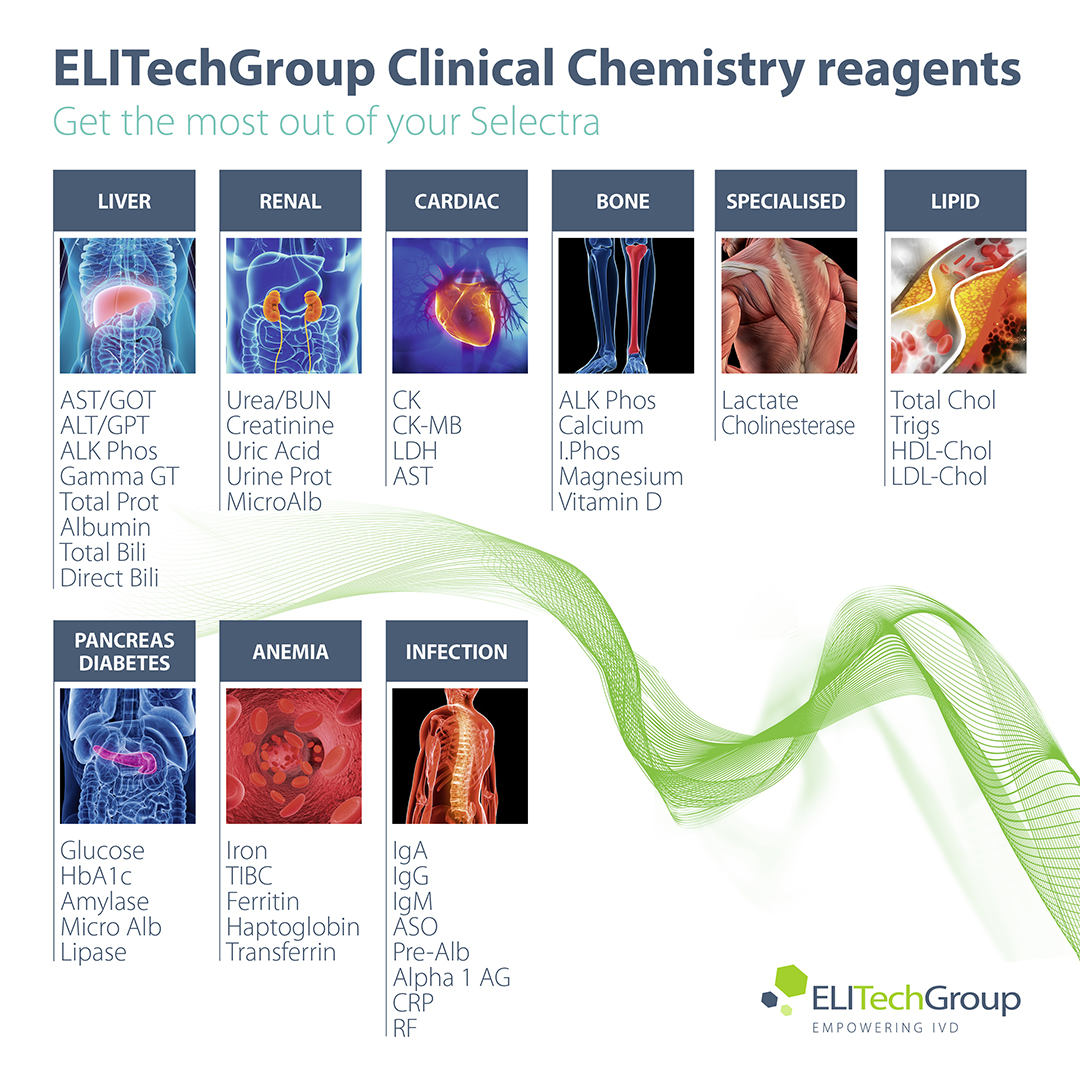
PRODUCT
The IQC, internal quality control, aims to safeguard consumers from harm, making quality control crucial in various high-standard industries, such as pharmaceuticals, automobiles, aviation, food and beverages, delivery services, electronics, and medical devices, including In-Vitro Diagnostics (IVD). A single mistake can result in severe consequences for the consumer, even life-threatening situations. In the clinical laboratory, imprecision can significantly affect clinical decisions. Good quality is invisible, but the bad quality is difficult to overlook.
In the clinical laboratory, Internal Quality Control (IQC) material mimics patient samples and contains multiple parameters for testing. Control materials (usually liquid controls) are used to monitor the test system and verify that quality patient test results have been attained. It can be pre-assayed or unassayed, with or without value ranges for each parameter. Assayed material is supplied with value ranges for each parameter included in this specific IQC material. For material supplied without value ranges for each parameter, the laboratory needs to establish its own QC reference ranges. IQC material can be a third-party quality control or a ‘System Supplier Control’ and then be part of the clinical chemistry system including calibrators, QC, instruments, reagents, and consumables from a complete integrated system. IQC should be run regularly as part of the daily workflow and at any time when something may have compromised the analytical quality.
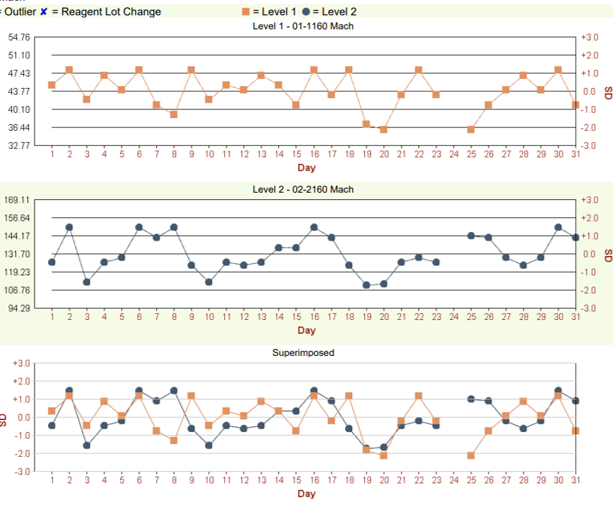
The clinical testing laboratory is responsible for ensuring and enhancing analytical quality to avoid significant impacts on clinical decisions due to imprecision. IQC testing, treating control materials like patient samples, is a routine part of the analytical process from sample placement to result generation. By comparing IQC results with established reference ranges and using QC statistical rules, the laboratory gains insights into accuracy and precision, helping to accept or reject patient results. IQC performance goals detect critical shifts in performance, preventing erroneous results and patient harm.
The first essential step in setting up internal quality control (IQC) of a test procedure in the clinical laboratory is to select the proper IQC procedure to implement. This includes choosing the statistical criteria or control rules, and the number of control measurements, according to the quality required for the test and the observed performance of the method. Then the right IQC procedure must be properly implemented.
Multirule QC uses a combination of decision criteria, or control rules, to decide whether an analytical run is in-control or out-of-control. The well-known Westgard multirule QC procedure uses 5 different control rules to judge the acceptability of an analytical run.
When an error is detected in IQC e.g., there’s a critical shift in performance or any of the IQC rules are violated, the following step is troubleshooting. A practical way is to establish a checklist to identify the root cause of the IQC issue.
Download a flyer ⇒ Calibrators and Quality Controls: Achieve accurate results and analytical excellence



For general inquiries, please use the links to the right. Click Contact to complete a brief online form, or click Support for general phone and email information. Someone will be in touch with you soon.